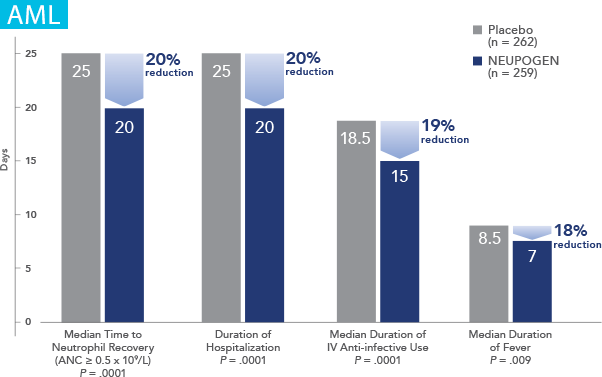
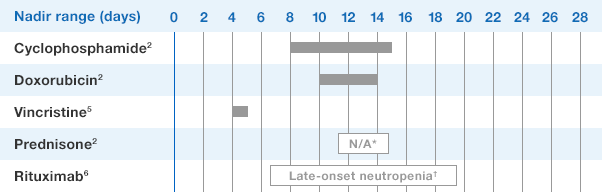
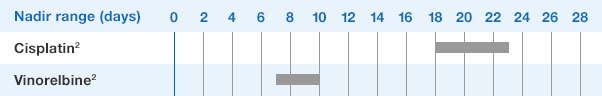
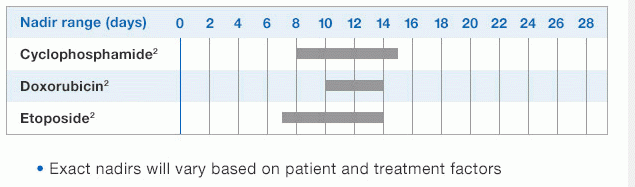
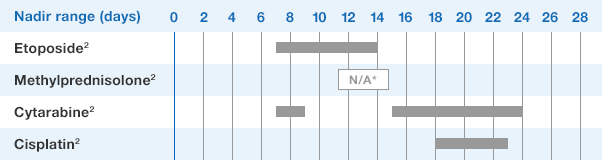
NEUPOGEN is indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with acute myeloid leukemia (AML).
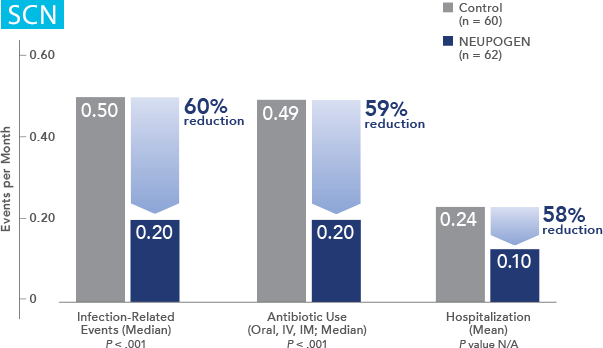
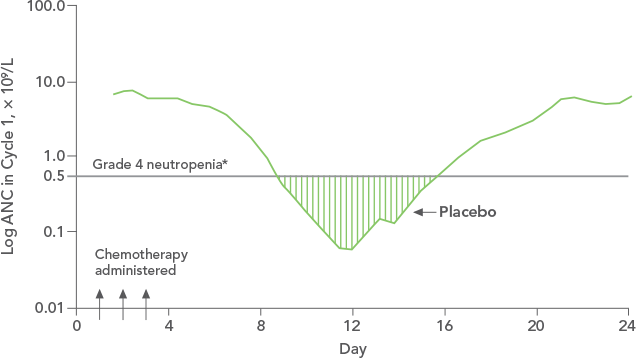
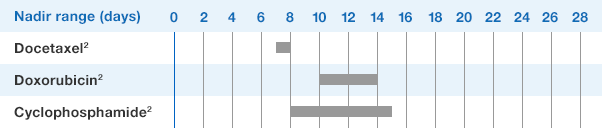
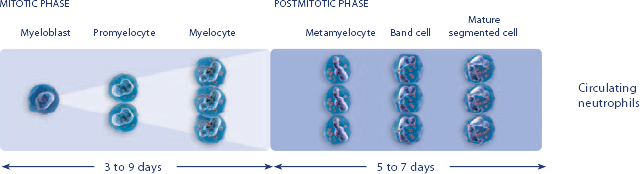
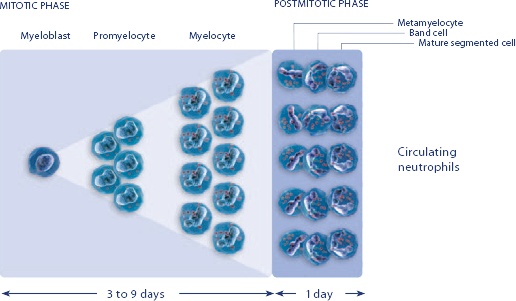
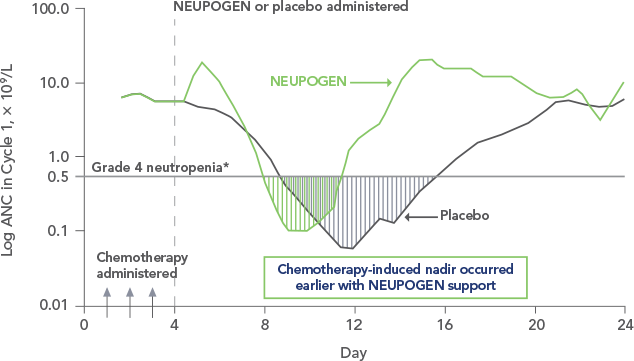
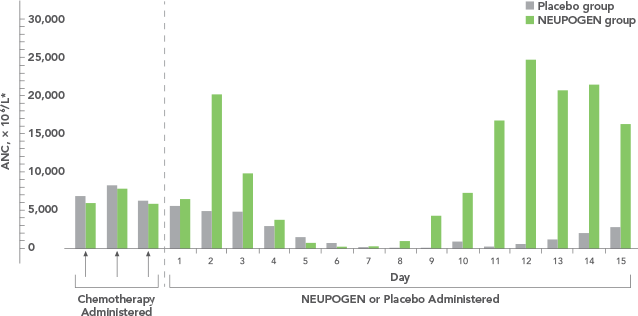
NEUPOGEN reduced the duration of neutropenic events in patients with acute myeloid leukemia receiving induction and consolidation chemotherapy2
ANC = absolute neutrophil count.
Study design: A prospective, multicenter, double-blind, randomized, placebo-controlled trial in patients with de novo AML receiving induction and consolidation therapy. Patients were centrally randomized after 6 days of chemotherapy and were stratified by center and age group (age < 50 years and age ≥ 50 years). Blinded study drug (NEUPOGEN 5 mg/kg/d or matched placebo) was given subcutaneously from 24 hours after the last dose of chemotherapy until the ANC was ≥ 1.0 × 109/L for 3 consecutive days or ≥ 10 × 109/L for 1 day. The primary objective of this study was to investigate the influence of filgrastim on disease outcome of de novo AML patients. Efficacy endpoints were the duration of neutropenia, the incidence and duration of fever and intravenous (IV) anti-infective therapy, the incidence of documented infections, and the duration of hospitalization.1
Heil G, et al. Blood. 1997;90:4710-4718.