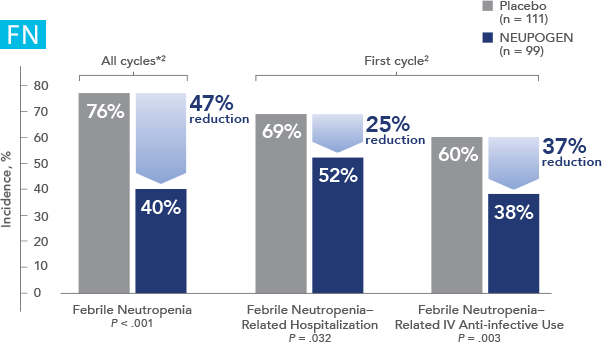
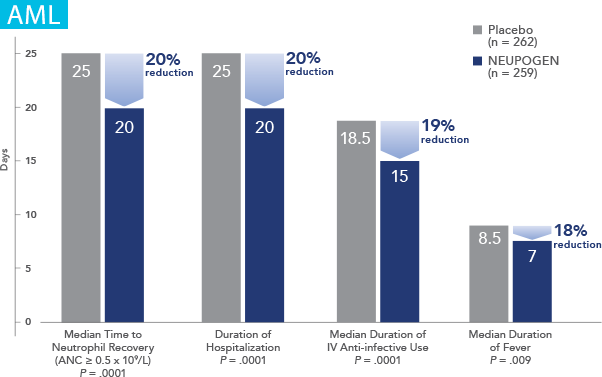
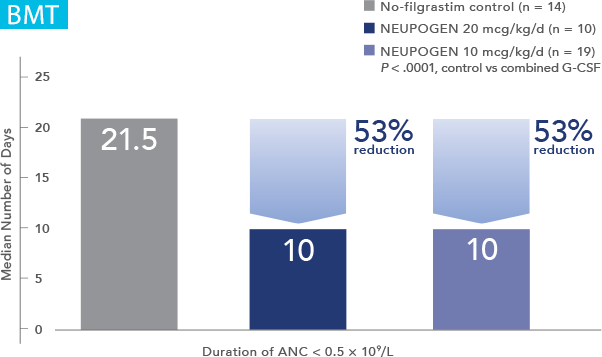
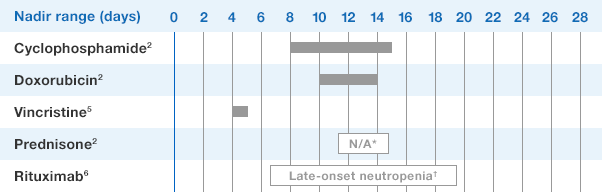
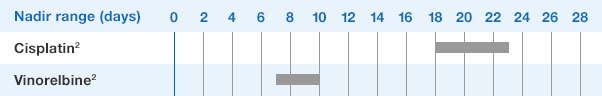
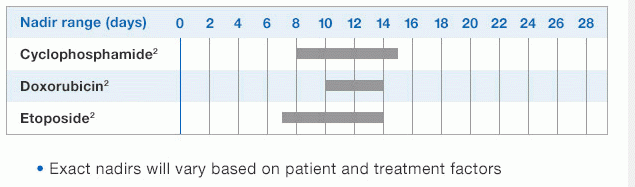
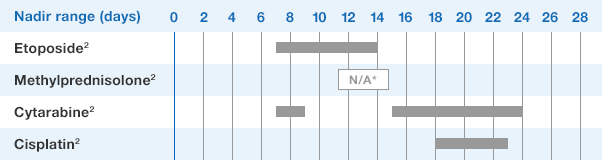
NEUPOGEN® is indicated for chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (e.g.‚ fever‚ infections‚ oropharyngeal ulcers) in symptomatic patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia.
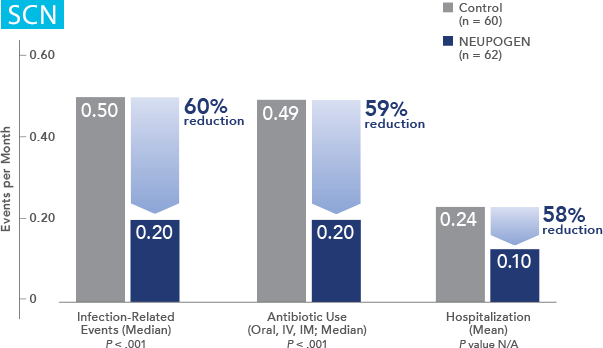
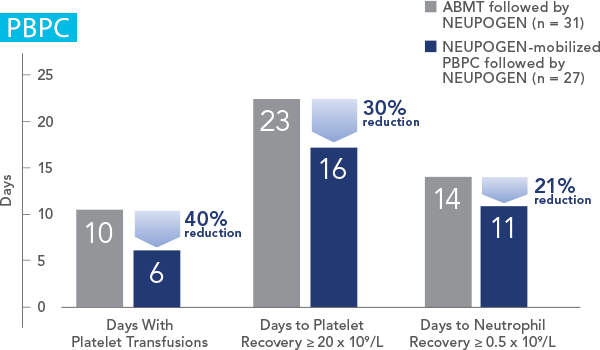
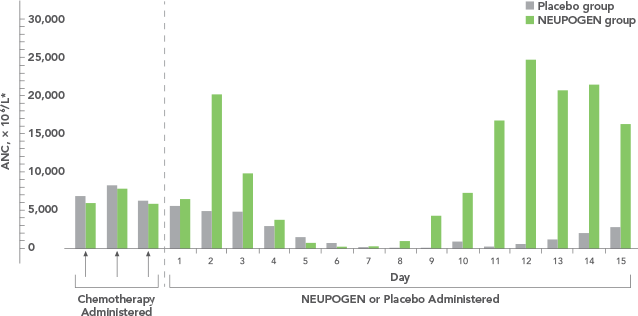
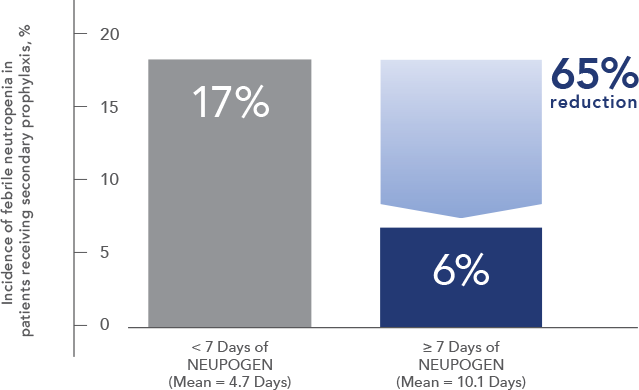
Study design: A randomized, placebo-controlled, multicenter phase 3 trial in adult and pediatric patients with SCN. Primary endpoint was to evaluate the efficacy of NEUPOGEN to increase the median ANC to ≥ 1.5 x 109/L; secondary endpoints included prevention/resolution of infections, hospitalizations, and antibiotic use. All results in the NEUPOGEN arm are from the 5-month treatment period and all results in the control arm are from the 4-month observation (no filgrastim) period. All results are expressed on a per month basis.2
NEUPOGEN® (filgrastim) Prescribing Information, Amgen. Dale CD, et al. Blood. 1993;81:2496-2502