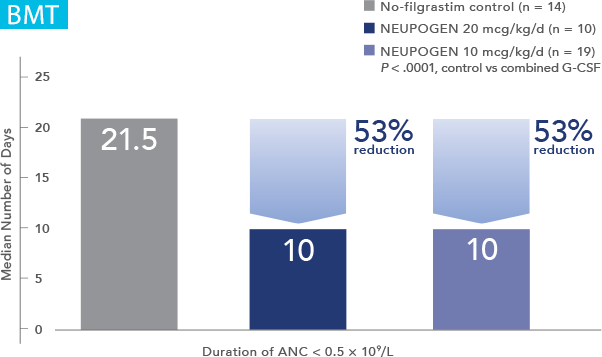
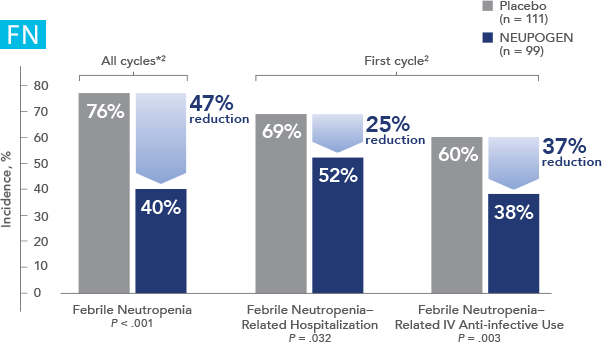
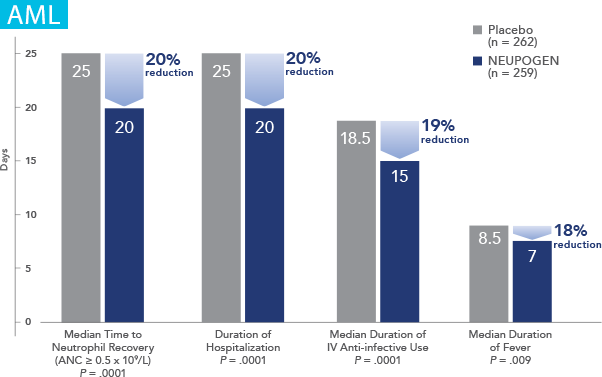
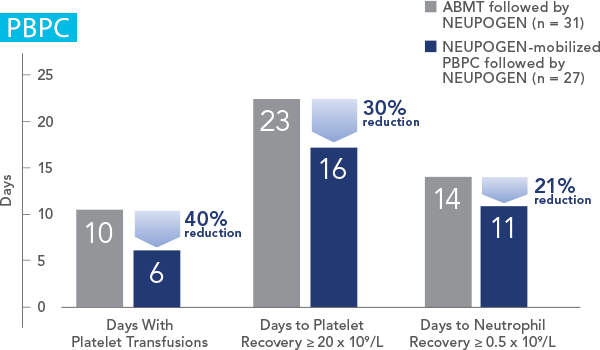
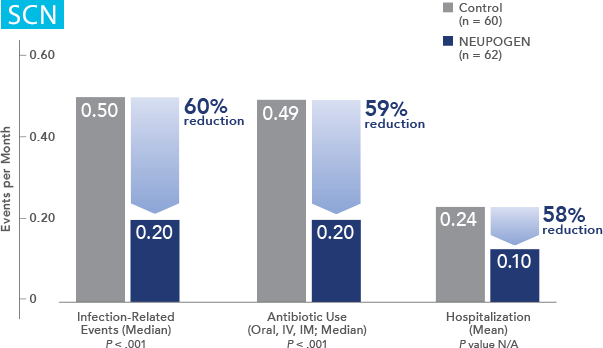
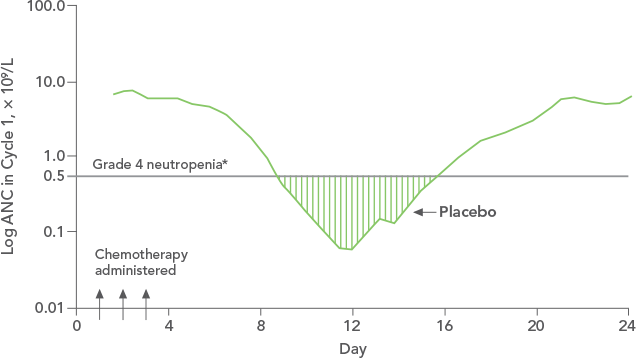
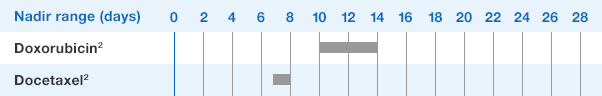
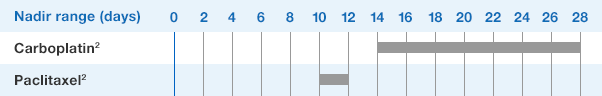
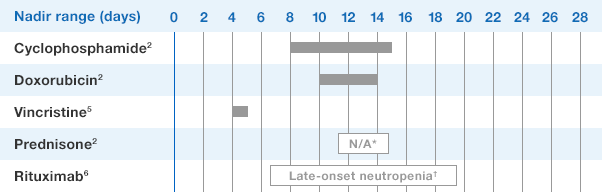
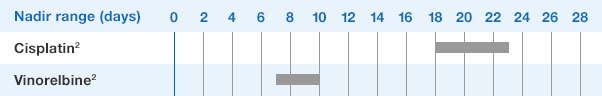
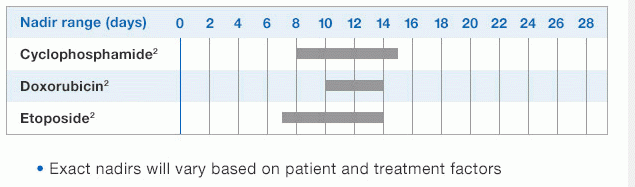
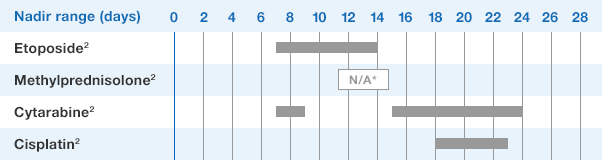
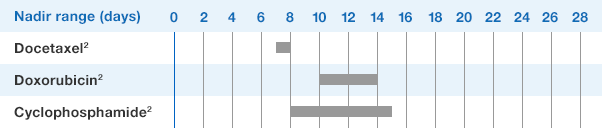
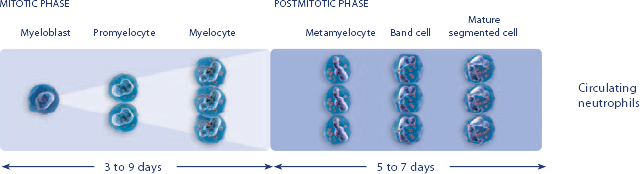
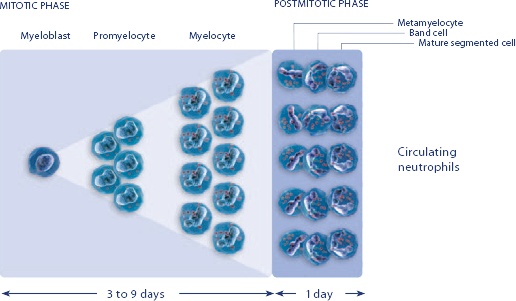
NEUPOGEN® is indicated to reduce the duration of neutropenia and neutropenia-related clinical sequelae‚ e.g. febrile neutropenia, in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplantation.
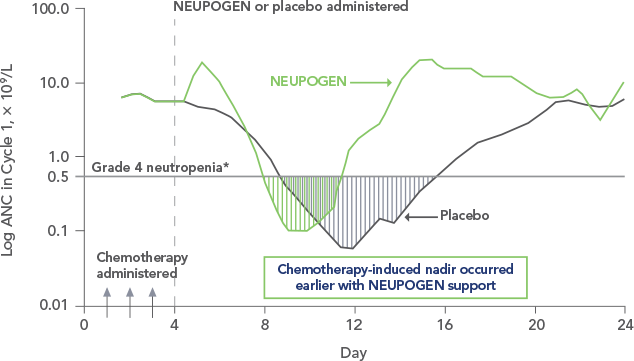
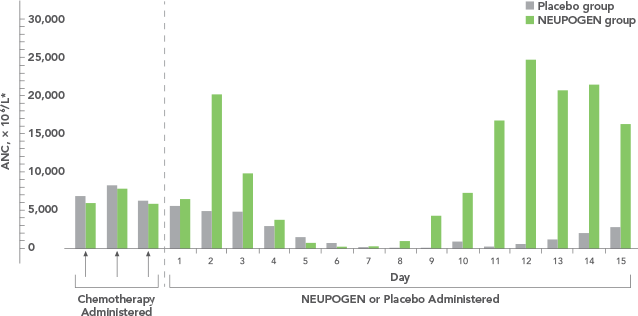
Study design: An open-label, prospective, randomized, controlled trial in patients with non-Hodgkin's lymphoma or relapsed Hodgkin's lymphoma receiving high-dose chemotherapy and autologous BMT. NEUPOGEN was administered daily for up to 28 days, starting on day 1 after bone marrow reinfusion, until ANC was > 1,0 x 109/L for 3 consecutive days after day 5. The aim of this trial was to examine the efficacy and safety of filgrastim after high-dose chemotherapy and autologous bone marrow transplantation (ABMT).1
Stahel RA, et al. J Clin Oncol. 1994;1994:12:1931-1938.