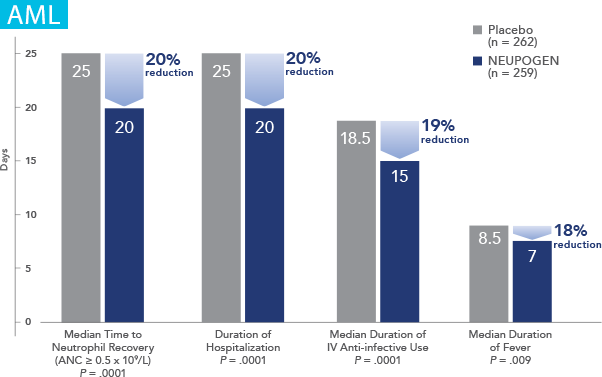
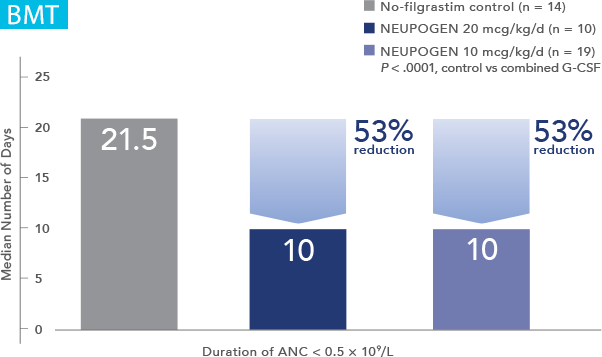
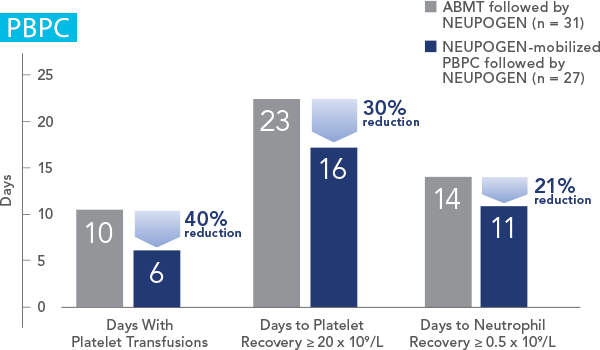
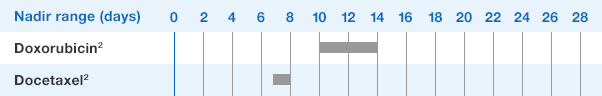
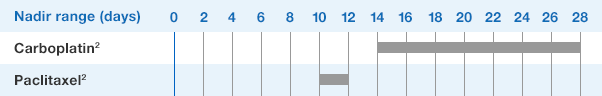
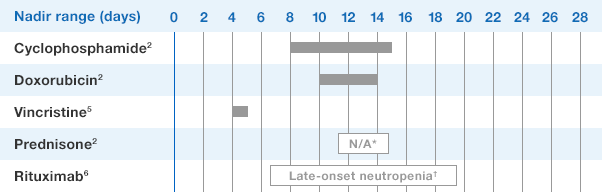
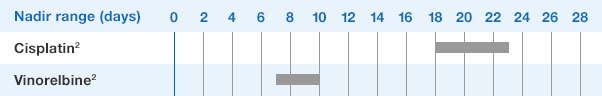
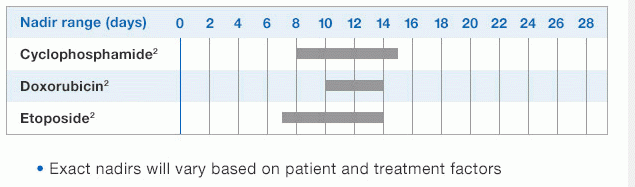
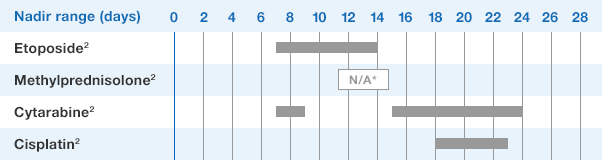
NEUPOGEN® is indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with acute myeloid leukemia (AML).
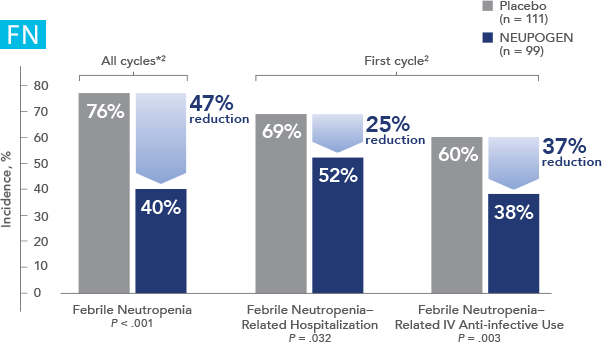
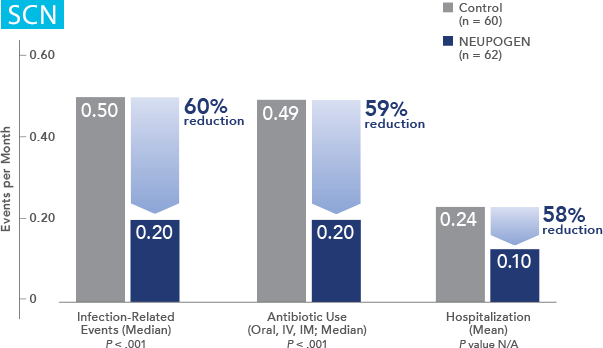
NEUPOGEN reduced the incidence of febrile neutropenia, hospitalization, and IV anti-infective use in patients receiving myelosuppressive chemotherapy1
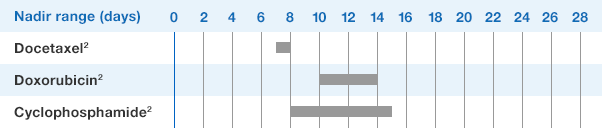
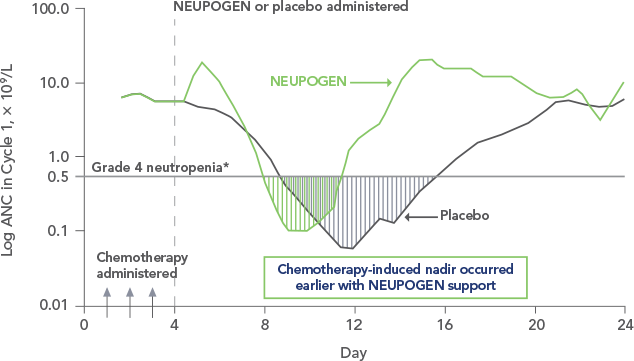
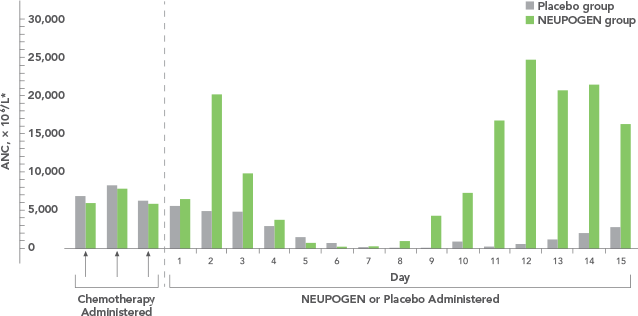
Study design: A multicenter, randomized, double-blind, placebo-controlled trial in patients with small-cell lung cancer receiving up to 6 cycles of chemotherapy with cyclophosphamide, doxorubicin, and etoposide. The intent-to-treat analysis included 210 patients who were randomized to receive either placebo (n=111) or NEUPOGEN (n=99) 24 hours post-chemotherapy (beginning on day 4 and continuing through day 12 and up to day 17 of a 21-day cycle unless the postnadir neutrophil count after day 12 exceeded 10.0 x 109/L, in which case growth factor was discontinued for the remainder of the cycle). Primary endpoint was the incidence of FN across the study duration; secondary endpoints included use of antibiotics and duration of hospitalization.1,2
Crawford J, et al. N Engl J Med. 1991;325:164-170. NEUPOGEN® (filgrastim) Prescribing Information, Amgen.
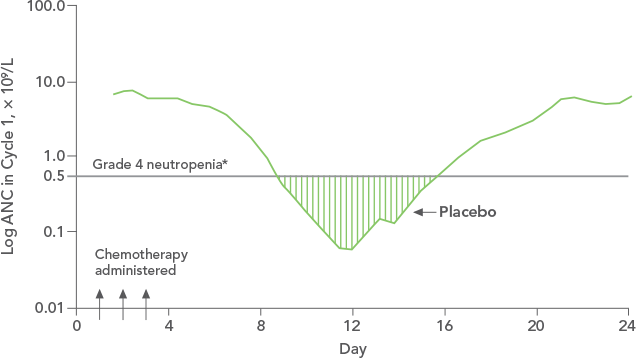
*FN = ANC < 1.0 x 109/L and oral temperature ≥ 38.2°C
FN = febrile neutropenia; ANC = absolute neutrophil count.