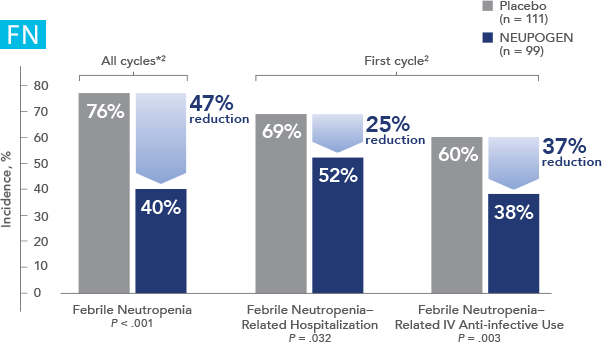
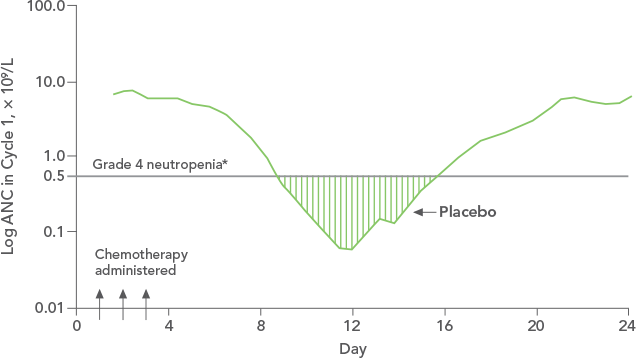
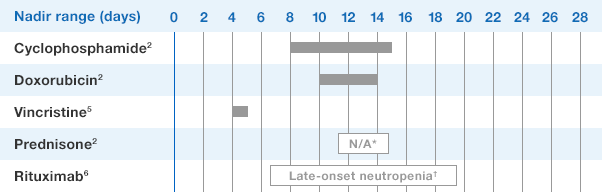
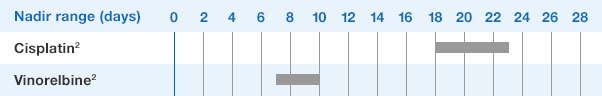
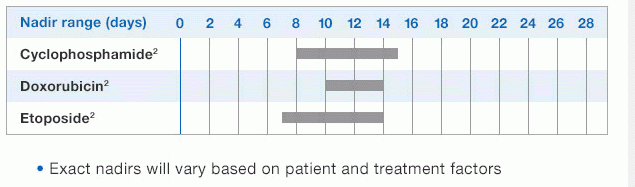
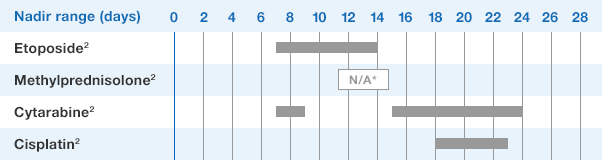
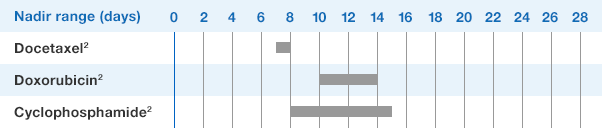
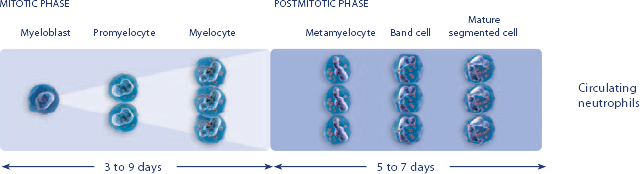
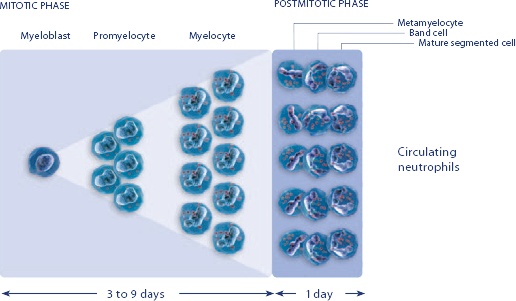
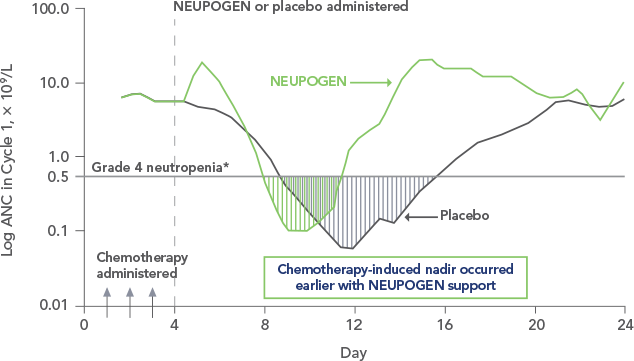
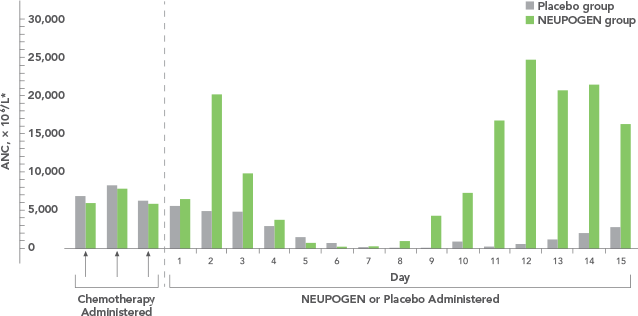
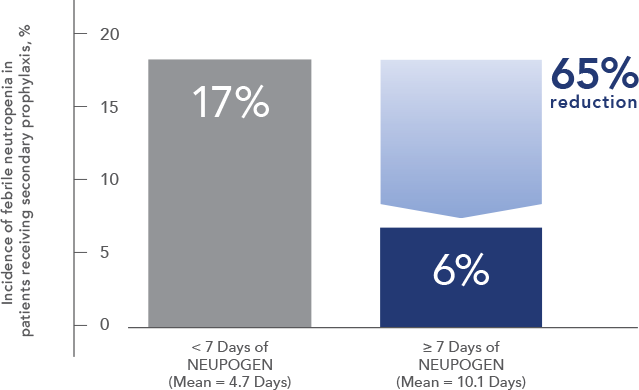

Study design: A multicenter, randomized, double-blind, placebo-controlled trial in patients with small-cell lung cancer receiving up to 6 cycles of chemotherapy with cyclophosphamide, doxorubicin, and etoposide. The intent-to-treat analysis included 210 patients who were randomized to receive either placebo (n=111) or NEUPOGEN (n=99) 24 hours post-chemotherapy (beginning on day 4 and continuing through day 12 and up to day 17 of a 21-day cycle unless the postnadir neutrophil count after day 12 exceeded 10.0 x 109/L, in which case growth factor was discontinued for the remainder of the cycle).1,2
Crawford J, et al. N Engl J Med. 1991;325:164-170. NEUPOGEN® (filgrastim) Prescribing Information, Amgen.
*Grade 4 neutropenia = ANC < 0.5 x 109/L.Contraindication
Splenic Rupture
Acute Respiratory Distress Syndrome (ARDS)
Serious Allergic Reactions
Sickle Cell Disorders
Glomerulonephritis
Alveolar Hemorrhage and Hemoptysis
Capillary Leak Syndrome (CLS)
Myelodysplastic Syndrome and Acute Myeloid Leukemia (AML)
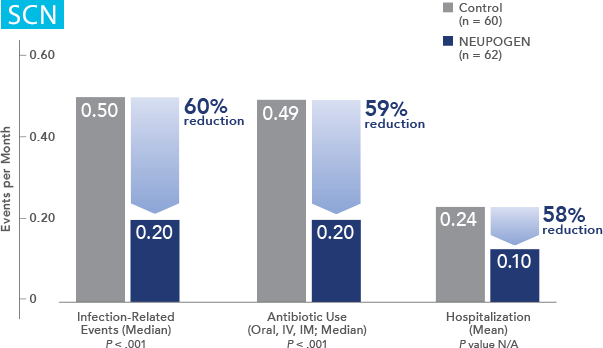
Patients with Severe Chronic Neutropenia
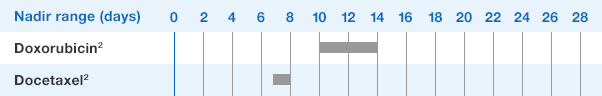
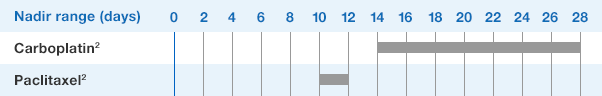
Patients with Breast and Lung Cancer
Thrombocytopenia
Leukocytosis
Patients with Cancer Receiving Myelosuppressive Chemotherapy:
Peripheral Blood Progenitor Cell Collection and Therapy (PBPC):
Cutaneous Vasculitis
Potential Effect on Malignant Cells
Simultaneous Use with Chemotherapy and Radiation Not Recommended
Nuclear Imaging
Aortitis
The most common adverse reactions in patients:
Please see NEUPOGEN® full Prescribing Information.
Patients with Cancer Receiving Myelosuppressive Chemotherapy
NEUPOGEN® is indicated to decrease the incidence of infection‚ as manifested by febrile neutropenia‚ in patients with nonmyeloid malignancies receiving myelosuppressive anti-cancer drugs associated with a significant incidence of severe neutropenia with fever.
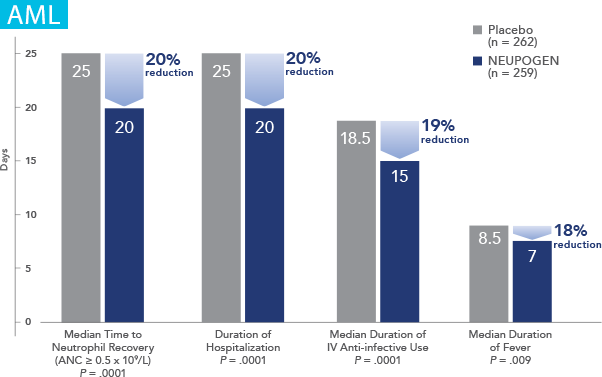
Patients with Acute Myeloid Leukemia Receiving Induction or Consolidation Chemotherapy
NEUPOGEN® is indicated for reducing the time to neutrophil recovery and the duration of fever, following induction or consolidation chemotherapy treatment of patients with acute myeloid leukemia (AML).
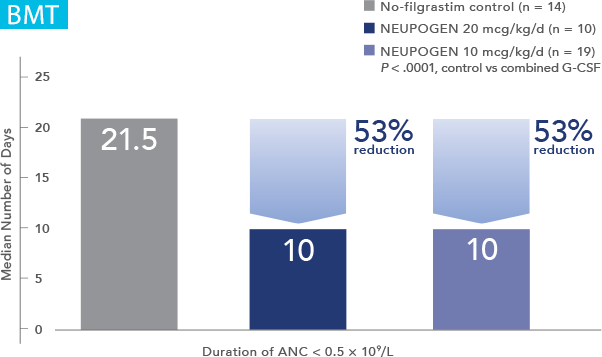
Patients with Cancer Undergoing Bone Marrow Transplantation
NEUPOGEN® is indicated to reduce the duration of neutropenia and neutropenia-related clinical sequelae‚ e.g. febrile neutropenia, in patients with nonmyeloid malignancies undergoing myeloablative chemotherapy followed by bone marrow transplantation.
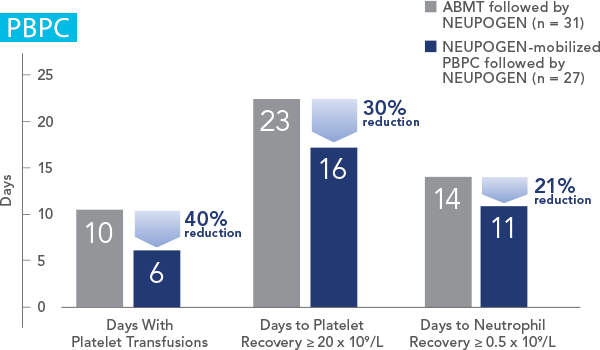
Patients Undergoing Autologous Peripheral Blood Progenitor Cell Collection and Therapy
NEUPOGEN® is indicated for the mobilization of autologous hematopoietic progenitor cells into the peripheral blood for collection by leukapheresis.
Patients with Severe Chronic Neutropenia
NEUPOGEN® is indicated for chronic administration to reduce the incidence and duration of sequelae of severe neutropenia (e.g.‚ fever‚ infections‚ oropharyngeal ulcers) in symptomatic patients with congenital neutropenia‚ cyclic neutropenia‚ or idiopathic neutropenia.
Contraindication
Splenic Rupture